ANTIBIOTICS CHEAT SHEET :)
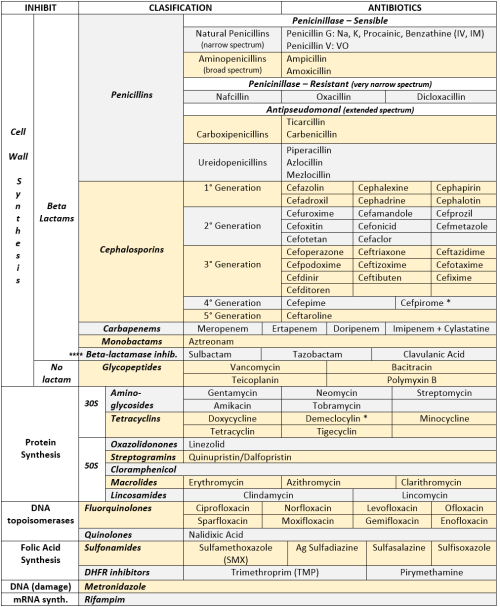
ANTIBIOTICS CHEAT SHEET :)
Also, REMEMBER!!!!
* Sulfonamides compete for albumin with:
Bilirrubin: given in 2°,3°T, high risk or indirect hyperBb and kernicterus in premies
Warfarin: increases toxicity: bleeding
* Beta-lactamase (penicinillase) Suceptible:
Natural Penicillins (G, V, F, K)
Aminopenicillins (Amoxicillin, Ampicillin)
Antipseudomonal Penicillins (Ticarcillin, Piperacillin)
* Beta-lactamase (penicinillase) Resistant:
Oxacillin, Nafcillin, Dicloxacillin
3°G, 4°G Cephalosporins
Carbapenems
Monobactams
Beta-lactamase inhibitors
* Penicillins enhanced with:
Clavulanic acid & Sulbactam (both are suicide inhibitors, they inhibit beta-lactamase)
Aminoglycosides (against enterococcus and psedomonas)
* Aminoglycosides enhanced with Aztreonam
* Penicillins: renal clearance EXCEPT Oxacillin & Nafcillin (bile)
* Cephalosporines: renal clearance EXCEPT Cefoperazone & Cefrtriaxone (bile)
* Both inhibited by Probenecid during tubular secretion.
* 2°G Cephalosporines: none cross BBB except Cefuroxime
* 3°G Cephalosporines: all cross BBB except Cefoperazone bc is highly highly lipid soluble, so is protein bound in plasma, therefore it doesn’t cross BBB.
* Cephalosporines are "LAME“ bc they do not cover this organisms
L isteria monocytogenes
A typicals (Mycoplasma, Chlamydia)
M RSA (except Ceftaroline, 5°G)
E nterococci

* Disulfiram-like effect: Cefotetan & Cefoperazone (mnemonic)
* Cefoperanzone: all the exceptions!!!
All 3°G cephalosporins cross the BBB except Cefoperazone.
All cephalosporins are renal cleared, except Cefoperazone.
Disulfiram-like effect
* Against Pseudomonas:
3°G Cef taz idime (taz taz taz taz)
4°G Cefepime, Cefpirome (not available in the USA)
Antipseudomonal penicillins
Aminoglycosides (synergy with beta-lactams)
Aztreonam (pseudomonal sepsis)
* Covers MRSA: Ceftaroline (rhymes w/ Caroline, Caroline the 5°G Ceph), Vancomycin, Daptomycin, Linezolid, Tigecycline.
* Covers VRSA: Linezolid, Dalfopristin/Quinupristin
* Aminoglycosides: decrease release of ACh in synapse and act as a Neuromuscular blocker, this is why it enhances effects of muscle relaxants.
* DEMECLOCYCLINE: tetracycline that’s not used as an AB, it is used as tx of SIADH to cause Nephrogenic Diabetes Insipidus (inhibits the V2 receptor in collecting ducts)
* Phototoxicity: Q ue S T ion?
Q uinolones
Sulfonamides
T etracyclines

* p450 inhibitors: Cloramphenicol, Macrolides (except Azithromycin), Sulfonamides
* Macrolides SE: Motilin stimulation, QT prolongation, reversible deafness, eosinophilia, cholestatic hepatitis
* Bactericidal: beta-lactams (penicillins, cephalosporins, monobactams, carbapenems), aminoglycosides, fluorquinolones, metronidazole.
* Baceriostatic: tetracyclins, streptogramins, chloramphenicol, lincosamides, oxazolidonones, macrolides, sulfonamides, DHFR inhibitors.
* Pseudomembranous colitis: Ampicillin, Amoxicillin, Clindamycin, Lincomycin.
* QT prolongation: macrolides, sometimes fluoroquinolones
More Posts from Fuadalanazi and Others
Penicillin

Penicillin is a widely used antibiotic prescribed to treat staphylococci and streptococci bacterial infections.
beta-lactam family
Gram-positive bacteria = thick cell walls containing high levels of peptidoglycan
gram-negative bacteria = thinner cell walls with low levels of peptidoglycan and surrounded by a lipopolysaccharide (LPS) layer that prevents antibiotic entry
penicillin is most effective against gram-positive bacteria where DD-transpeptidase activity is highest.
Examples of penicillins include:
amoxicillin
ampicillin
bacampicillin
oxacillin
penicillin
Mechanism(s)
Penicillin inhibits the bacterial enzyme transpeptidase, responsible for catalysing the final peptidoglycan crosslinking stage of bacterial cell wall synthesis.
Cells wall is weakened and cells swell as water enters and then burst (lysis)
Becomes permanently covalently bonded to the enzymes’s active site (irreversible)


Alternative theory: penicillin mimics D-Ala D-Ala

Or may act as an umbrella inhibitor

Resistance
production of beta-lactamase - destroys the beta-lactam ring of penicillin and makes it ineffective (eg Staphylococcus aureus - most are now resistant)
In response, synthetic penicillin that is resistant to beta-lactamase is in use including egdicloxacillin, oxacillin, nafcillin, and methicillin.
Some is resistant to methicillin - methicillin-resistant Staphylococcus aureus (MRSA).
Demonstrating blanket resistance to all beta-lactam antibiotics -extremely serious health risk.



Cone snails might not seem like deadly predators, especially when you consider how easily a fish could outswim them. However, these snails await the cover of darkness to prey on sleeping fish. They appear to release paralyzing chemicals before using a venomous barb to finally put the fish out of its misery. (Source)

يالله 💙

No bacterium is an island.
Many people think of bacteria as tiny Lone Rangers, paddling their flagellar canoes across the desolate petri dish sea. But in “the wild”, bacteria exist as complex, interwoven, constantly competing social communities.
Every scoop of soil is a battlefield of chemical chatter. Species send out molecular messages-in-a-bottle that ride the waves of diffusion to their mates. Some even thread electrical cables between neighboring cells. Now, new research has identified elaborate shared membranes that let single cells swarm as a superorganism …
Check out my latest article for Wired all about a soil bacterium named Myxococcus xanthus. It’s under everyone’s feet right now, and it has developed one of the most elaborate physical webs ever witnessed in bacteria. That’s it up top, devouring a colony of E. coli using its patented rippling wave attack.
It’s a stealth communication network that lets them hunt like a tiny wolfpack. So cool. Plus I got to use a GIF, so double win.
Once you’re done with that, check out this great TED talk from Bonnie Bassler all about how bacteria communicate.

Frankenstein’s…bacteriophages?
A group of scientists have just published their work in CellPress on synthesising novel phages using common viral structures (Figure 1), to target a range of bacterial hosts. Anyone who frequently reads my posts will be fully aware of what bacteriophages are, however put simply phages are viruses that target bacteria (Figure 1). Currently a lot of research is going into these little guys to utilise them for both therapeutic and diagnostic purposes.

Figure 1. Image depicts a transmission electron micrograph of a phage (left) and a diagrammatic representation of the phage on the (right) showing all the major components that make up the protein coat.
Phages have an elaborate protein coat that surrounds their DNA cargo (contained within the head, Figure 1). They scan their surrounding environment for potential ‘prey’ by using their tail fibres to interact with their cognate receptors. The diversity of receptors is immense and many still go unidentified even for well characterised phages. Once a target is found, the baseplate then irreversibly attaches to the cell wall of the bacteria and the DNA can be transferred into the cytoplasm of the cell.
Unlike current therapeutics, the sheer diversity between phages means that they are highly specific with limited host ranges to the species or even strain level. As a result, for phage therapy to be effective, a cocktail of phages needs to be employed to target a wide range of potential bacterial hosts. To improve the host ranges of phage groups such as this one are trying to synthesis novel structures from existing phages - much like how Frankenstein stitched together a new human body from pre-existing parts (Figure 2).

Figure 2. Simplified image showing how bacterial components can be shuffled between genomes of related phages with different host ranges.
Innovatively, this group used a well characterised yeast-based (Saccharomyces cerevisiae) platform for capturing phage genomes to allow their genetic manipulation (Figure 3). Phage genomes could then be inserted into a yeast artificial chromosome (YAC) then manipulated. Yeast are fairly easy to genetically modify by homologous-recombingation, making this system far easier to employ than other methods. The YAC was then recovered and transformed into the normal host bacteria, allowing the generation of new phage particles. Unlike other methods phage generated via this method were relatively easy to reboot back into active viral particles.

Figure 3. Illustration of the genetic manipulation of phage DNA to generate hybrid phages with altered host ranges.
The group managed to show that by swapping modular components such as tail fibres between phages, new host specificity could be generated. Thus illustrating that gene swapping can overcome strain or species barriers if the need arises. This work will hopefully lead way to further improve phage therapy and decrease the persistent need for the identification of novel phages. Further work needs to be done on increasing the scope of their work, but they have created a framework that will hopefully be able to reboot more synthetic phages.
“Our results show that common phage scaffolds can be re-targeted against new bacterial hosts by engineering single or multiple tail components. This capability enables the the construction of defined phage cocktails that only differ in their host range determinants and can be used to edit the compositions of microbial consortia and/or treat bacterial infections.” - Ando et al, 2015
Sources:
Engineering Modular Viral Scaffolds for Targeted Bacterial Population Editing, 2015. Ando, H., Lemire. S., Pires. D.P., Lu. T.K., Cell Systems , Volume 1 , Issue 3 , 187 - 196

امين يارب


This week’s infographic is a metabolic map showing the chemical reactions that happen inside our body. You can check out the full size version here.
تأثير سماع القرآن على الكافر ..
-
 2025bs2026 liked this · 2 months ago
2025bs2026 liked this · 2 months ago -
 ant-heia liked this · 3 months ago
ant-heia liked this · 3 months ago -
 tooearthquakekoala liked this · 6 months ago
tooearthquakekoala liked this · 6 months ago -
 tmarkmulles liked this · 7 months ago
tmarkmulles liked this · 7 months ago -
 sunfortune liked this · 9 months ago
sunfortune liked this · 9 months ago -
 crimsonrebel liked this · 9 months ago
crimsonrebel liked this · 9 months ago -
 muwanguzi-01 liked this · 10 months ago
muwanguzi-01 liked this · 10 months ago -
 okbro-wegetit liked this · 10 months ago
okbro-wegetit liked this · 10 months ago -
 adennnsstuff liked this · 11 months ago
adennnsstuff liked this · 11 months ago -
 daddysbunnypixie liked this · 1 year ago
daddysbunnypixie liked this · 1 year ago -
 allecrax liked this · 1 year ago
allecrax liked this · 1 year ago -
 micazin liked this · 1 year ago
micazin liked this · 1 year ago -
 jingerlei liked this · 1 year ago
jingerlei liked this · 1 year ago -
 perla-estrada-universe liked this · 1 year ago
perla-estrada-universe liked this · 1 year ago -
 quantumdoctor15 liked this · 1 year ago
quantumdoctor15 liked this · 1 year ago -
 cerebrology reblogged this · 1 year ago
cerebrology reblogged this · 1 year ago -
 hopenada reblogged this · 1 year ago
hopenada reblogged this · 1 year ago -
 hopenada liked this · 1 year ago
hopenada liked this · 1 year ago -
 semie78 liked this · 1 year ago
semie78 liked this · 1 year ago -
 ngugikamau liked this · 1 year ago
ngugikamau liked this · 1 year ago -
 atomicstarburstlabware reblogged this · 1 year ago
atomicstarburstlabware reblogged this · 1 year ago -
 yahajbaba liked this · 1 year ago
yahajbaba liked this · 1 year ago -
 louis7473 liked this · 1 year ago
louis7473 liked this · 1 year ago -
 hellohiomg-blog reblogged this · 1 year ago
hellohiomg-blog reblogged this · 1 year ago -
 hellohiomg-blog liked this · 1 year ago
hellohiomg-blog liked this · 1 year ago -
 basicallyarganine liked this · 1 year ago
basicallyarganine liked this · 1 year ago -
 provokinq liked this · 1 year ago
provokinq liked this · 1 year ago -
 pabsitiltogis liked this · 1 year ago
pabsitiltogis liked this · 1 year ago -
 conkecounpimpflow liked this · 1 year ago
conkecounpimpflow liked this · 1 year ago -
 wtrsine88 liked this · 1 year ago
wtrsine88 liked this · 1 year ago -
 ticserupma liked this · 1 year ago
ticserupma liked this · 1 year ago -
 maincarescha liked this · 1 year ago
maincarescha liked this · 1 year ago -
 styllenhardwen liked this · 1 year ago
styllenhardwen liked this · 1 year ago -
 gonsuheci liked this · 1 year ago
gonsuheci liked this · 1 year ago -
 webedragons liked this · 1 year ago
webedragons liked this · 1 year ago -
 studyhard2studyabroad reblogged this · 1 year ago
studyhard2studyabroad reblogged this · 1 year ago -
 kuromegami liked this · 1 year ago
kuromegami liked this · 1 year ago